Abstract
Introduction. Ibrunitib (IBR) is active in chronic lymphocytic leukemia (CLL) patients (pts) with TP53 aberrations. Few data describing the dynamics of TP53 mutated clones under IBR are available. We analyzed a cohort of 40 treatment-naïve and relapsed CLL pts treated with IBR to investigate the dynamics of clonal and subclonal TP53 mutations (TP53-mut).
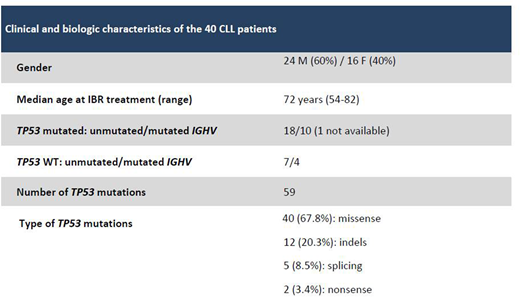
Methods. Forty pts (Table) underwent a longitudinal TP53 monitoring (117 samples) by ultra-deep sequencing (UDS): 26 received IBR + rituximab (IBR+RTX) in first line as part of the GIMEMA LLC 1114 protocol (IBR exposition: 8 months in 7 pts and 14 months in 19 pts) (cohort 1), while 14 received IBR single agent after a median of 1.5 (range: 1-4) chemo-immunotherapy lines (IBR exposition: 2.1 to 4 years in 12 pts) (cohort 2). Samples were analyzed by UDS on a MiSeq sequencer (Illumina, Inc.) to obtain a 5000X coverage/base. For variant calling, the MiSeq Reporter software and an in-house bioinformatics pipeline were applied. All mutations were checked on the IARC TP53 database and those with a variant allele frequency (VAF) <10% (i.e. subclonal) were confirmed in an independent UDS run. VAF was corrected to cancer cell fraction (CCF) by the proportion of CD19+/CD5+ cells.
Results. In cohort 1, 12/26 pts were evaluated at 3 time-points: baseline (T0), +8 (T8) and +14 (T14) months from IBR+RTX, and 14 at T0 and either T8 or T14. At T0, 19/26 pts showed a mean number of 1.5 (range: 1-5) clonal/subclonal TP53-mut/pt, for a total of 28 mutations. Of those, 20/28 (71.4%) were clonal (mean VAF: 57.8%; range: 18-94.8%) and 8/28 (27.6%) were subclonal (mean VAF: 4.4%; range: 1.2-9.2%; VAF≤5% in 6). Seven/26 pts resulted wild-type (WT). Under IBR+RTX, of the 28 TP53-mut corrected to CCF (21 clonal and 7 subclonal), 12 (9 clonal + 3 subclonal) (42.8%) persisted stable, 9 (32.1%) clonal mutations decreased, 6 (21.4%) were lost, one evolved to clonal. No novel clonal or subclonal TP53-mut arose during IBR+RTX.
According to CCF, the pts followed 5 patterns: 1) clonal TP53-mut present from T0 and persisting clonal with a stable (n=6) or decreasing CCF (n=7); 2) clonal TP53-mut disappearing during treatment (n=1); 3) subclonal TP53-mut evolving to clonal (n=1, CCF 8% at T0 and 17.5% at T14); 4) subclonal TP53-mut persisting subclonal (n=1); 5) absence of any detectable TP53-mut in all time-points (n=7). In addition, 3 cases showed coexisting clonal and subclonal TP53-mut at T0: in one case 3 TP53-mut remained stable; in another one, 4 TP53-mut, including one clonal, were lost, and one clonal decreased in CCF; in the last case, 1 TP53-mut decreased, 1 remained stable and 1 subclonal disappeared.
In cohort 2, before IBR, 10/14 pts showed a mean of 3.1 (range: 1-11) clonal/subclonal TP53-mut/pt, for a total of 31 mutations. Of those, 11/31 (35.5%) were clonal (mean VAF: 31.9%; range: 10.5-78.8%) and 20/31 (64.5%) were subclonal (mean VAF: 2.9%; range: 0.9-6.8%). Four/14 pts were WT.
Under IBR, 16/31 (6 clonal+10 subclonal) (51.5%) TP53-mut persisted stable, 2 (6.5%) clonal decreased, 11 (2 clonal+9 subclonal) (35.5%) were lost, 2 (6.5%) subclonal evolved to clonal; 2 novel subclonal mutations emerged. No mutation was identified in the 4 WT pts over time.
In both cohorts, most of TP53-mut remained stable (42.8% vs 51.5% in cohort 1 and 2, respectively) or decreased (32.1% vs 6.5%) and 17 (5 clonal and 12 subclonal) were lost (21.4% vs 35.5%) (p=NS). Although the lymphocyte count significantly decreased during IBR+RTX/IBR exposure (cohort 1: 47.1 x 109/L vs 7.5 x 109/L, p<0.0001; cohort 2: 48.5 x 109/L vs 15.3 x 109/L, p=0.015), the mean CCF of the existing mutations remained stable on treatment (cohort 1: 48.1% vs 40.1%, p=0.42; cohort 2: 16.9 % vs 13.02%; p=0.5).
Conclusions. Both when used front-line or as a subsequent line of therapy, IBR appears to decrease the TP53 clonal and subclonal numerosity and complexity. Clonal evolution and the occurrence of novel mutations are rare and occur mostly in pre-treated pts. The significant decrease of lymphocytosis with stable CCF, prove the IBR effectiveness both on TP53 mutated and WT CLL cells, regardless of previous therapies. A longer follow-up will better clarify the dynamics of clonal and subclonal TP53-mut and whether the persistent clones may survive over time and give rise to subsequent relapses.
Mauro:abbvie: Other: board member; janssen: Other: board member. Foà:GILEAD: Speakers Bureau; CELTRION: Other: ADVISORY BOARD; INCYTE: Other: ADVISORY BOARD; ROCHE: Other: ADVISORY BOARD, Speakers Bureau; JANSSEN: Other: ADVISORY BOARD, Speakers Bureau; NOVARTIS: Speakers Bureau; CELGENE: Other: ADVISORY BOARD, Speakers Bureau; AMGEN: Other: ADVISORY BOARD; ABBVIE: Other: ADVISORY BOARD, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal